Growing demand for high-capacity batteries has led to extensive exploration of Lithium-Sulfur (LiS) battery technology. While the theoretical potential of LiS batteries is substantial, practical applications have faced challenges such as slow electrochemical reactions and sulfur loss during charge/discharge cycles.
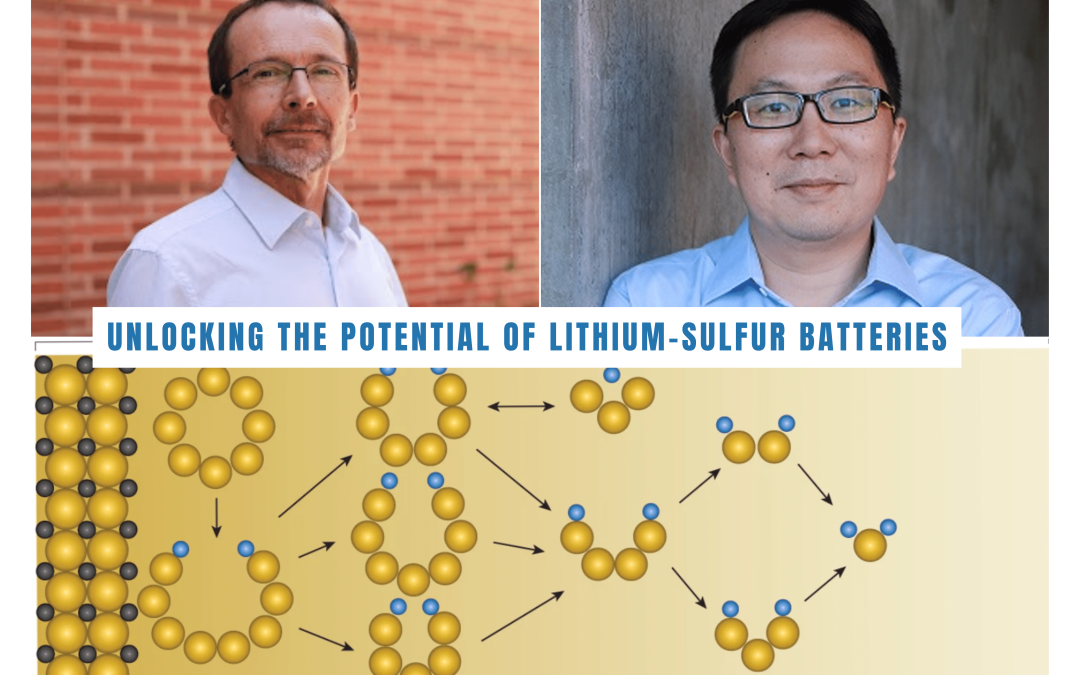
Funded by the Center for Synthetic Control Across Length-scales for Advancing Rechargeables, an Energy Frontier Research Center funded by the U.S. Department of Energy Office of Science Basic Energy Sciences program, UCLA Professors Philippe Sautet and Xiangfeng Duan have focused on deciphering the complexities of the sulfur reduction reaction in LiS batteries. Published in the journal Nature, their findings offer insights into the intricate reaction network involving 16 electrons and various intermediate products. The researchers successfully mapped out the dominant molecular pathway and highlighted the critical role of electrocatalysis in modifying the reaction kinetics.
The research delves into the sulfur reduction reaction in LiS batteries, aiming to uncover pathways for improved performance. The key to enhancing LiS battery capacity lies in catalysis, a process that accelerates charging reactions, allowing for a more complete charge and significantly increasing the battery’s overall capacity. The study identifies Li2S4 as a crucial intermediate in the sulfur reduction reaction, with its efficient conversion being vital for battery performance. The team discovered that carbon-based electrodes doped with sulfur and nitrogen serve as highly effective catalysts for this conversion.
These collective efforts underline the potential breakthroughs that arise from the synergy between battery technology and catalysis science. As society seeks fast and high-capacity energy conversion devices, these findings pave the way for advancements in LiS battery technology.